In-vitro Fertilization (IVF)
What is IVF?
In-vitro fertilization and embryo transfer (IVF), also known as ‘test-tube baby’, is an effective treatment for infertility. It involves collecting eggs from a woman’s ovaries, fertilizing the eggs with a man’s sperm in the laboratory, culturing these fertilized eggs to become embryos and returning them into the woman’s womb. It was originally developed to help women with blocked fallopian tubes to conceive, but has since been expanded to treat couples with various causes of infertility.
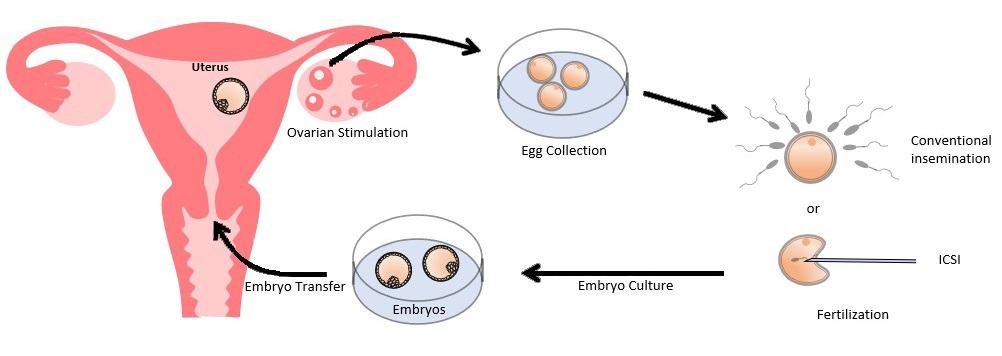
Schematic diagram of IVF treatment
 scroll right to see more
scroll right to see more
Who may need IVF?
Women with blocked or damaged fallopian tubes;
Men with poor semen quality;
Women with severe endometriosis;
Women in their advanced reproductive age or low ovarian reserve, as time to conception is critical;
All other causes of infertility including unexplained infertility, especially if treatment with other methods has failed;
Couples with an inherited genetic disease that they wish to avoid passing on to their child, and in this case, IVF is combined with pre-implantation genetic testing (PGT) to select embryos without the disease for transfer.
What does IVF involve?
(I) Ovarian Stimulation
(II) Egg Collection
(III) Fertilization
(IV) Embryo Culture
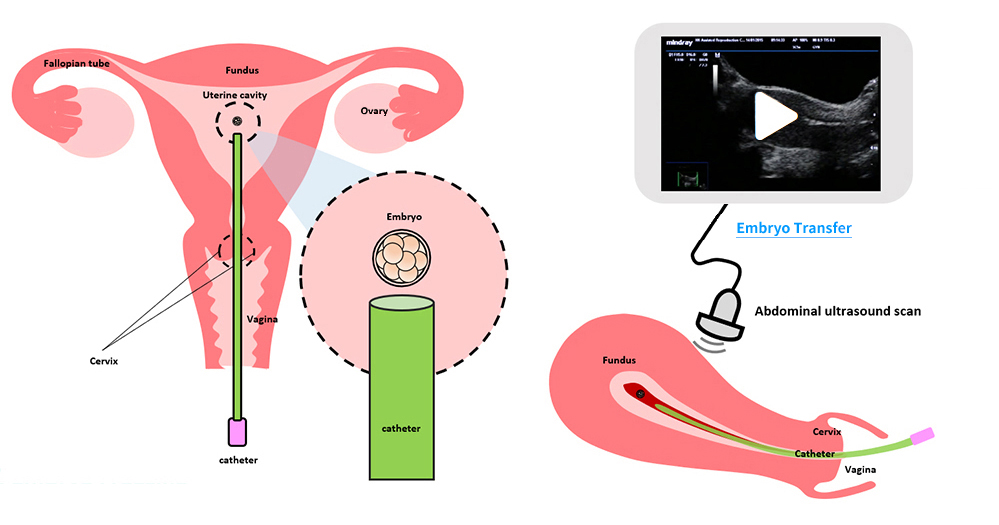
(V) Embryo Transfer
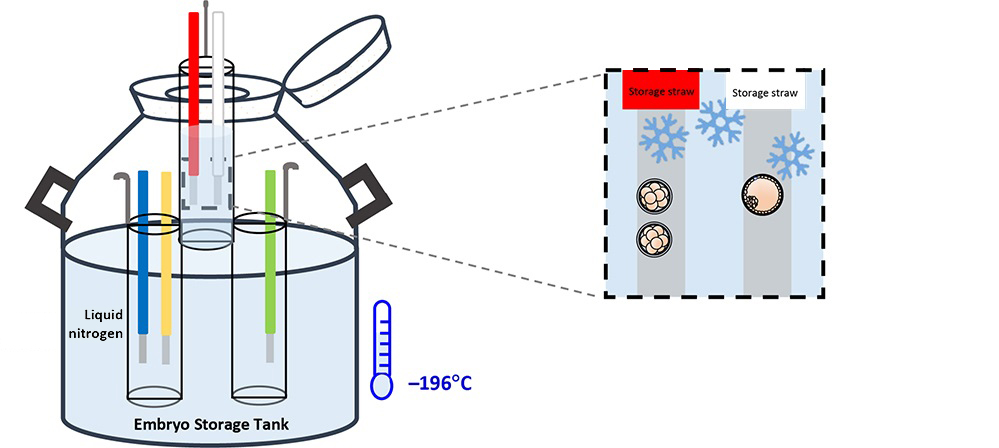
(VI) Embryo Freezing
(I) Ovarian Stimulation
In order to collect more eggs, fertility drugs are used to stimulate the ovaries. There are different stimulation protocols. The most commonly used one is the antagonist protocol.
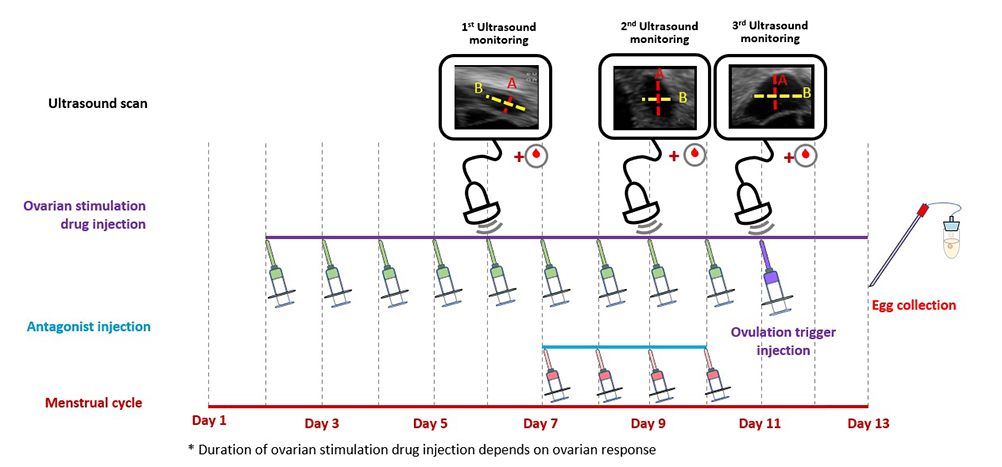
 scroll right to see more
scroll right to see more
Antagonist protocol: The woman takes daily ovarian stimulation drug injections in the beginning of her cycle to boost the growth of follicles (or eggs). Another injection drug, known as an antagonist, is added in the latter part of the stimulation period to prevent the eggs from being released too soon. The woman needs ultrasound scans and blood tests to monitor the growth of the follicles. When the eggs are ready, an ovulation trigger injection is given to complete the final maturation of the eggs and egg collection can be arranged.
Other less commonly used protocols include: |
|
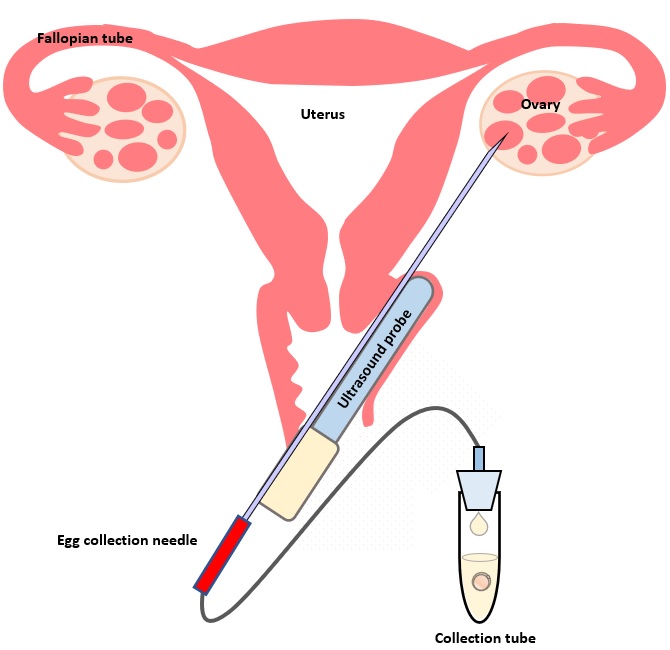
(II) Egg Collection
The egg collection procedure is performed under ultrasound guidance. An ultrasound probe is inserted into the vagina to identify the follicles. A thin needle is then inserted into an ultrasound guide to go through the vagina and into the follicle. The follicular fluid is aspirated and collected into a test tube. An embryologist then looks at the follicular fluid under a stereomicroscope to collect the eggs.
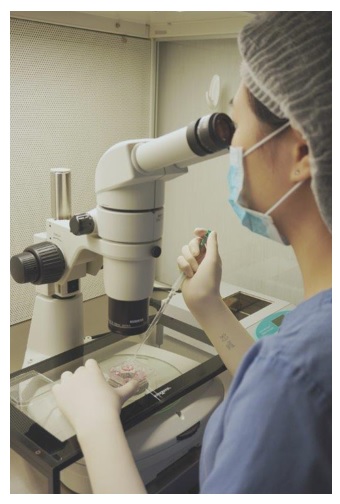
An embryologist is looking down a stereomicroscope, checking follicular fluid to see if an egg can be found.
The egg collection procedure takes around 30–45 minutes. It is a painful procedure, and therefore anaesthesia is given. The woman can go home after resting in the IVF centre for a few hours, and return to work the next day.
(III) Fertilization
The man has to produce a semen sample on the day of the egg collection. The semen sample is processed, and the sperm are used for insemination with one of the following methods:
-
Conventional insemination
Conventional insemination is a process by which the sperm and eggs are mixed together, and the sperm penetrate the eggs by themselves. This insemination method is suitable for couples with adequate active and normal sperm.
-
Intracytoplasmic sperm injection (ICSI)
Intracytoplasmic sperm injection (ICSI) is a procedure involving the direct injection of a single sperm into an egg to assist fertilization. ICSI is usually performed in couples with specific indications:
Severe male factor infertility;
Previous IVF using conventional insemination had none or very few eggs fertilized;
Planned PGT;
Frozen-thawed sperm that are not as active.
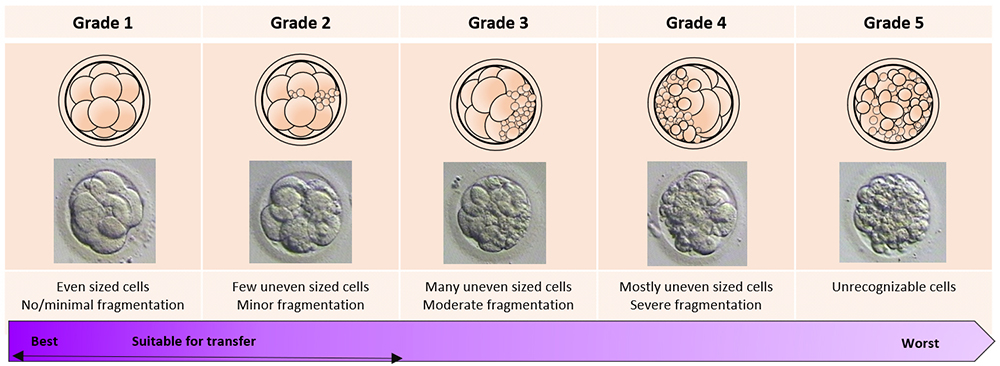
(IV) Embryo Culture
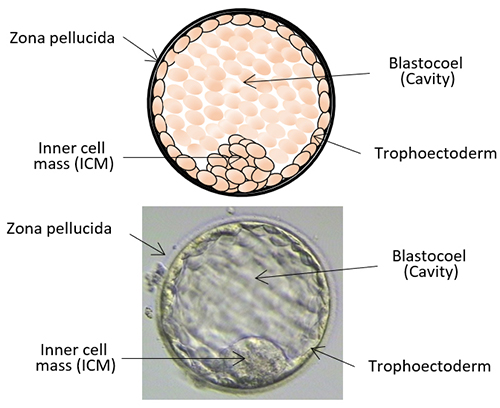
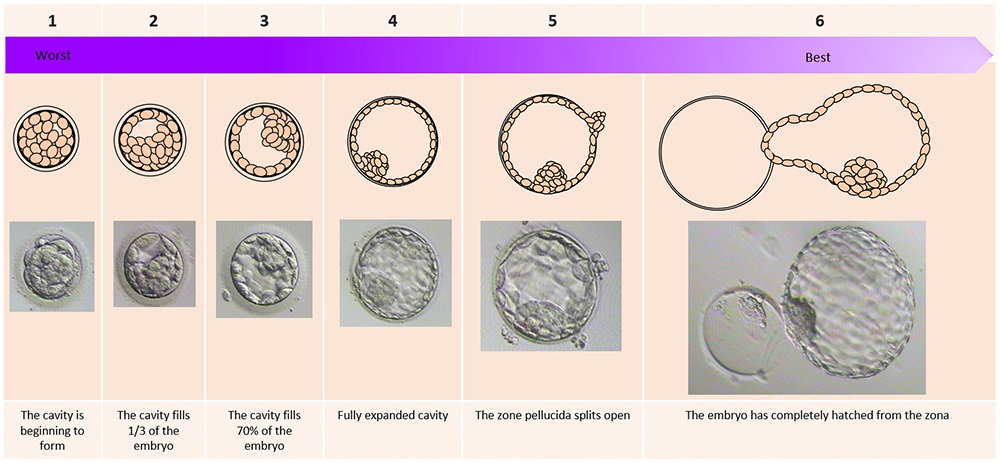
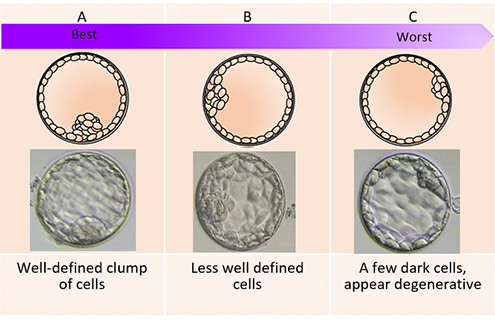
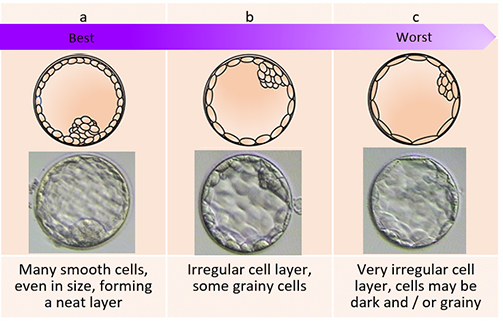
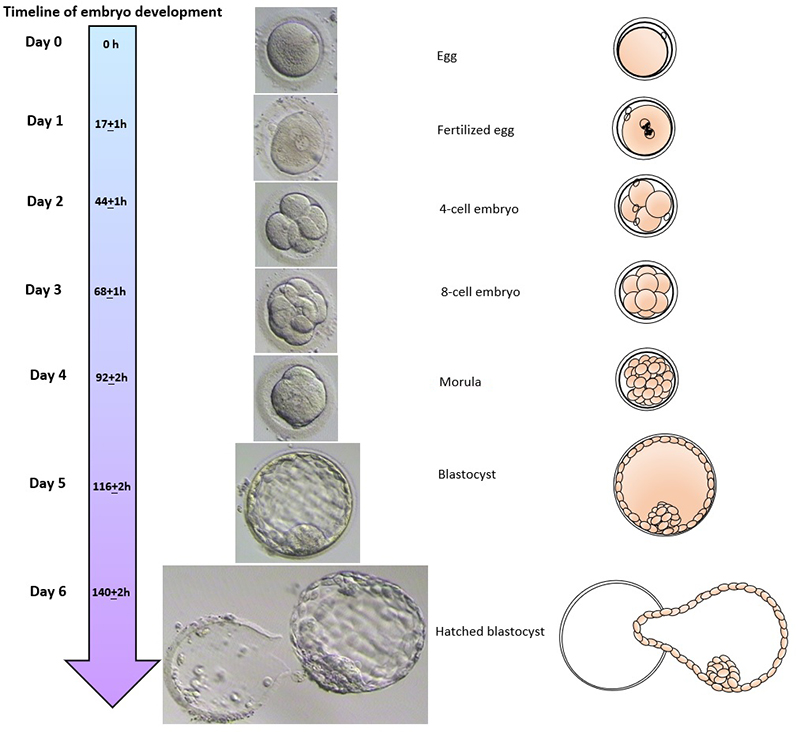
The day of the egg collection is called Day 0 in the timeline of embryo development. On Day 1, embryologists check the eggs to see if fertilization is successful. On Day 2, the fertilized eggs undergo cell division and become embryos. The embryos can be cultured for up to 5 days when they are called blastocysts. They can be transferred back into the uterus on either Day 2, Day 3 or Day 5.
Embryo development
 scroll right to see more
scroll right to see more